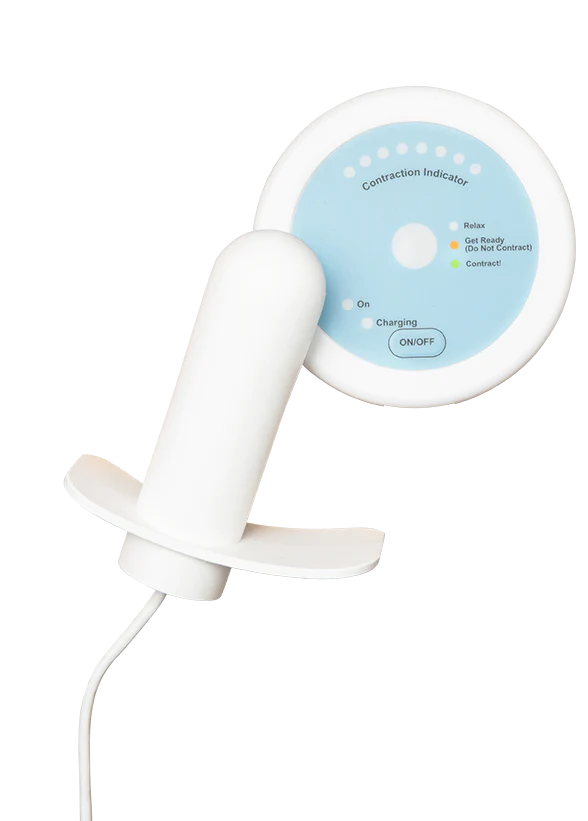
Surgical-level results. Without surgery.
Clinically proven to treat stress urinary incontinence.
Treat the cause, not the symptoms
Flyte® is the only at-home intravaginal device that delivers the proven therapeutic treatment modality of mechanotherapy to treat stress urinary incontinence and strengthen the pelvic floor. Flyte® is the first and only treatment of its kind.
How Flyte Works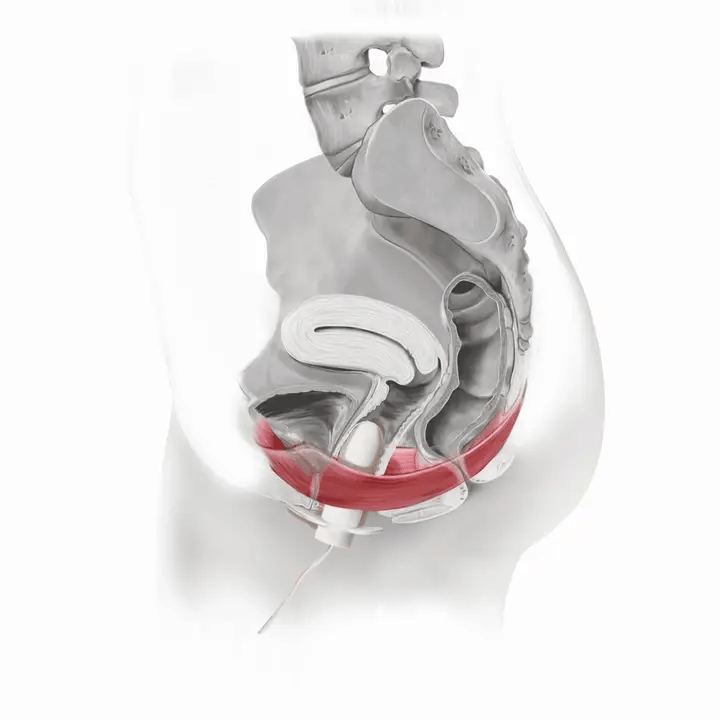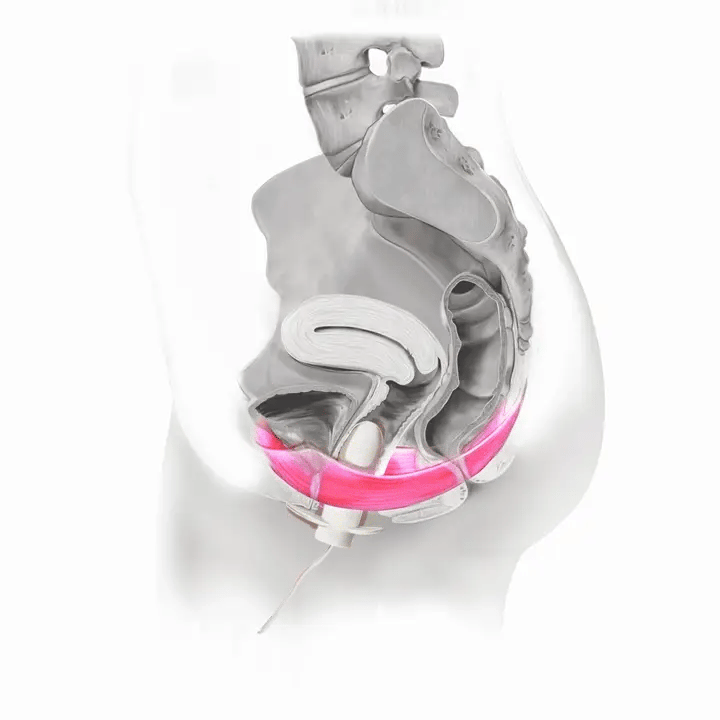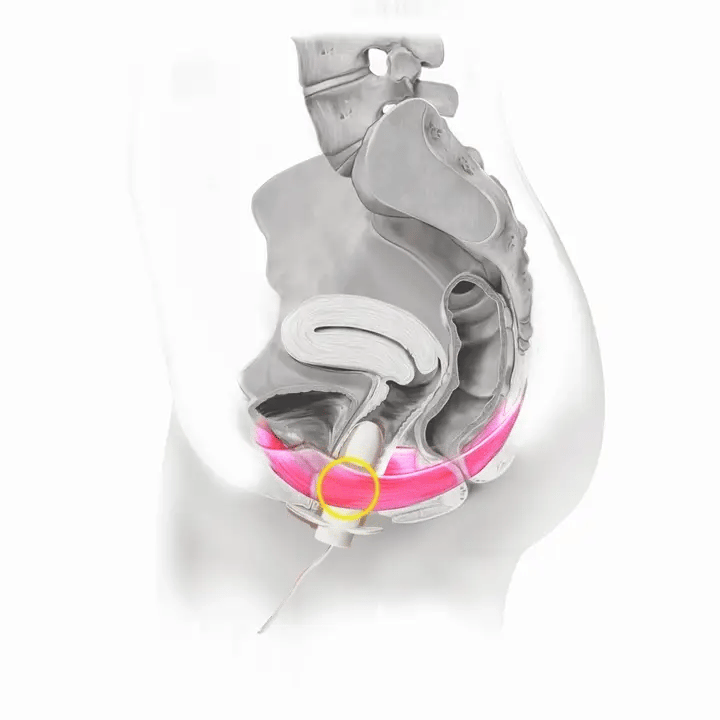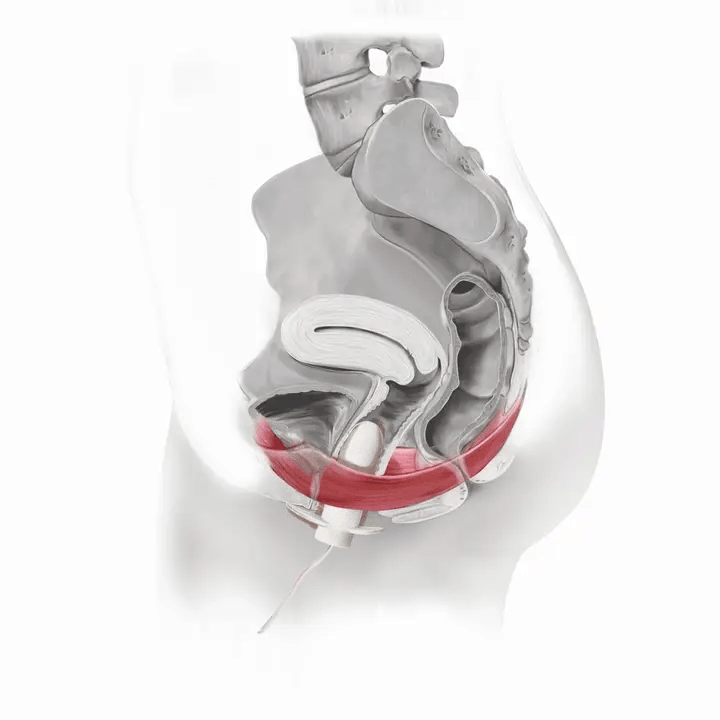

Patient Supported
"I used to have bladder leaks EVERY time I exercised, sneezed, coughed, etc. Now, I have very little to no leakage. This is life changing!" - Lisa, Flyte user

Clinically Proven
82% of women were continent in 6 weeks

FDA Cleared
Flyte® is FDA cleared and is backed by over 20 years of research and development including rigorous clinical studies.

Recommended by Healthcare Providers
"Definitely consider this FDA-cleared Flyte therapy as an alternative to surgery." - Dr. Nissirine Nakib, MD
Evidence Backed
Flyte® was studied in patients with stress urinary incontinence (SUI) in two key studies including a 60 patient study in Norway and a 119 patient study in the U.S., one of the largest of its kind for an in-home SUI treatment. Both studies used the objective measure of pad weight as the primary outcome measure. Both studies also looked at quality of life and long-term durability (whether the treatment lasts even after you stop using Flyte).
The Science
Easy to Use
Inserting Flyte is simple, like inserting a tampon. Each 5-minute session delivers a series of gentle mechanical vibrations while you contract and relax your pelvic floor.

Comfortable
The Flyte wand is covered in smooth medical grade silicone to make insertion comfortable.

Expert Support
Talk to a Doctor of Physical Therapy pelvic health specialist to guide you through your journey to continence.
What Women are Saying
FAQs
Flyte® is easy to use.
The Flyte® wand is covered in smooth medical-grade silicone to make insertion easy. We recommend applying a water-based vaginal lubricant to the wand if needed to make insertion easier.
Flyte® does not use electrical stimulation and your treatment should not be painful. During your treatment cycle you will feel Flyte's gentle vibrations.
You may experience some discomfort when you first begin to use an intravaginal device. This is normal and is similar to some of the muscle fatigue or soreness you might feel after starting any new exercise program.
Flyte® is designed to work fast — in as little as 5 minutes per day for 6 weeks. Some women may require a 12-week treatment or may benefit from continued use of Flyte®.
Each woman is different, and some women find they need continued treatment beyond our standard treatment time (6-12 weeks). After your initial treatment, you may use Flyte® as directed by your clinician, as needed to meet your personal goals, or to maintain pelvic floor muscle tone.
The first step is to locate your pelvic floor muscle. One technique is to imagine you are trying to keep yourself from passing gas. Or, imagine tensing your muscles as if to stop peeing mid-stream. The muscles you use are your pelvic floor muscles.
Once you’ve identified the muscle, squeeze and hold: Imagine you are trying to keep a marble from falling out of your vagina. Think about lifting and pulling in. Don’t push down. Try not to activate other muscles in your abdomen, thighs or butt. Visualize a lifting motion and breathe in and out. Don’t hold your breath. Contract these muscles for three seconds. Now relax them for three seconds. You’ve just performed a Kegel exercise.
The Flyte® wand has sensors inside it that measure the tone of your pelvic floor muscles at rest and when you squeeze. The wand transmits this information to the handheld controller -- in other words, it tells you when to relax and contract (squeeze and lift) your pelvic floor muscles.
With each cycle, focus on the timing and coordination of your pelvic floor muscles. The blue lights reflect how well you are squeezing and lifting your pelvic floor muscles in comparison to your muscles at rest.
If you activate these lights (even very few!) and follow along, you know you are using Flyte® and engaging your pelvic floor muscles correctly.
This is how Flyte® teaches you how to properly activate, coordinate, and strengthen your pelvic floor. At the same time, Flyte’s® gentle mechanical vibrations (mechanotherapy) are amplifying the benefit of your squeezes (Kegels) by 39 times (data on file at Pelvital).
If you would like to see if Flyte is covered by your insurance plan, please contact Flyte Customer Care at support@flytetherapy.com or call 866-735-8482 (press 1).
If you have money in a flexible spending account (FSA) or a health savings account (HSA) through your employer-based health insurance, you may be able to use this money to help pay for and purchase Flyte.
No. Flyte® is not electrical stimulation (e-stim) and is not a Kegel trainer.
There are no electrodes and Flyte® does not force your muscles to contract through electrical stimulation, which some women find uncomfortable.
Instead, Flyte® uses a technology called mechanotherapy to leverage the body’s natural healing process to improve and restore the pelvic floor muscles, giving you a stronger pelvic floor.
Flyte® is an active treatment, backed by clinical evidence and is FDA cleared.
Flyte® leverages the body’s natural process to heal and restore the pelvic floor muscles. The cells in our body are designed to sense change in our environment and then respond to that change. The Flyte® wand is designed to make contact with the pelvic floor, providing a gentle, therapeutic stretch on the muscles. Second, the Flyte® wand delivers gentle mechanical vibrations to stimulate the pelvic floor. These two steps work together to create a cellular response in the pelvic floor by generating improved strength and tone – giving you more control over your bladder.
From the blog
View all1. Nilsen I, Rebolledo G, Acharya G, Leivseth G. Mechanical oscillations superimposed on the pelvic floor muscles during Kegel exercises reduce urine leakage in women suffering from stress urinary incontinence: A prospective cohort study with a 2-year follow up. Acta Obstet Gynecol Scand. 2018 Oct;97(10):1185-1191. doi: 10.1111/aogs.13412. Epub 2018 Aug 2. PMID: 29923602.
2. Labrie J, Berghmans BL, Fischer K, Milani AL, van der Wijk I, Smalbraak DJ, Vollebregt A, Schellart RP, Graziosi GC, van der Ploeg JM, Brouns JF, Tiersma ES, Groenendijk AG, Scholten P, Mol BW, Blokhuis EE, Adriaanse AH, Schram A, Roovers JP, Lagro-Janssen AL, van der Vaart CH. Surgery versus physiotherapy for stress urinary incontinence. N Engl J Med. 2013 Sep 19;369(12):1124-33. doi: 10.1056/NEJMoa1210627. PMID: 24047061.
3. Yao J, Tse V. Twenty-Five Years of the Midurethral Sling: Lessons Learned. Int Neurourol J. 2022 Jun;26(2):102-110. doi: 10.5213/inj.2142086.043. Epub 2022 Jun 30. PMID: 35793988; PMCID: PMC9260325.
4. Rogo-Gupta L, Baxter ZC, Le NB, Raz S, Rodríguez LV. Long-term durability of the distal urethral polypropylene sling for the treatment of stress urinary incontinence: minimum 11-year followup. J Urol. 2012 Nov;188(5):1822-7. doi: 10.1016/j.juro.2012.07.033. Epub 2012 Sep 19. PMID: 22999687.
5. Nakib N, Sutherland S, Hallman K, Mianulli M, R Boulware D. Randomized trial of mechanotherapy for the treatment of stress urinary incontinence in women. Ther Adv Urol. 2024 Feb 6;16:17562872241228023. doi: 10.1177/17562872241228023. PMID: 38328552; PMCID: PMC10848796.